Antibody Variable Regions: Structure, Function, and Applications
What is an Antibody Variable Region?
The antibody molecule consists of two main regions: the constant region and the variable region. The variable region, located at the tips of the antibody, is composed of parts of the heavy (VH) and light (VL) chains. These regions are responsible for the antigen-binding specificity and diversity of the antibody.
The primary function of the variable region is to bind to a specific antigen. The unique amino acid sequence in this region allows each antibody to recognize a distinct epitope on an antigen, making it a crucial component in the adaptive immune response.
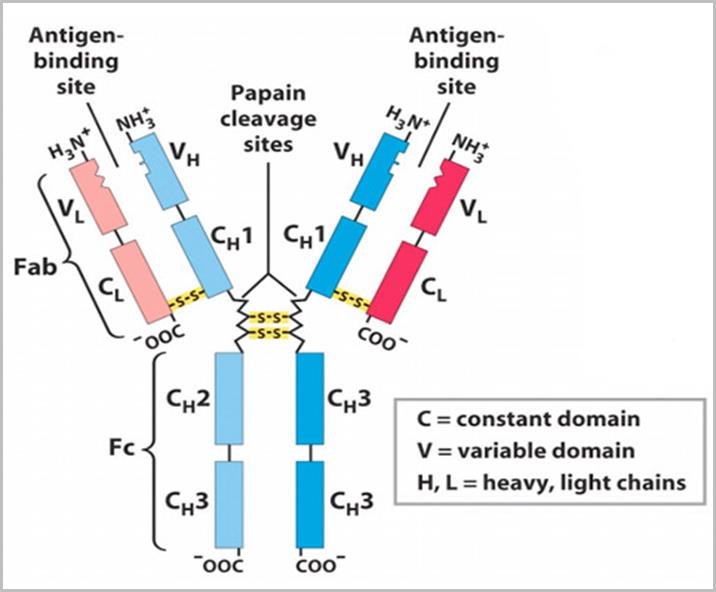
Showing the variable and constant region of an antibody and the antigen binding sites (Mir et al., 2020).
Structural Characteristics of the Variable Region
The antibody molecule is Y-shaped, consisting of four polypeptide chains: two identical heavy (H) chains and two identical light (L) chains. Each chain is subdivided into variable (V) and constant (C) regions. The variable regions of the heavy and light chains (VH and VL, respectively) are located at the tips of the Y-shaped molecule and form the antigen-binding site.
The variable region is so named because of its highly diverse amino acid sequence, which allows antibodies to recognize a vast array of antigens. The VH and VL domains each contain approximately 110-130 amino acids and together form a unique antigen-binding site.
Complementarity-Determining Regions (CDRs)
Within the variable region, there are specific areas known as Complementarity-Determining Regions (CDRs). These are the most variable parts of the antibody and are critical for antigen binding. Each variable region (VH and VL) has three CDRs: CDR1, CDR2, and CDR3. These regions create the surface that directly interacts with the antigen.
- CDR1 and CDR2: Located in the variable domain and contribute to forming the antigen-binding site. Their sequences are relatively less variable compared to CDR3.
- CDR3: This region is the most variable and often plays the most significant role in determining the specificity of antigen binding. It is formed by the joining of V, D (for heavy chains), and J gene segments during the process of V(D)J recombination.
Framework Regions (FRs)
Surrounding the CDRs are the framework regions (FRs), which are more conserved and provide structural support to the variable region. These regions ensure the proper folding and stability of the antibody molecule, maintaining the correct positioning of the CDRs to interact with antigens effectively.
Generation of Diversity
The remarkable diversity of the antibody variable region works through several mechanisms:
V(D)J Recombination
During B cell development, the variable region genes undergo a process called V(D)J recombination. This involves the random combination of variable (V), diversity (D), and joining (J) gene segments for the heavy chain, and V and J segments for the light chain. This combinatorial process generates a vast repertoire of unique variable regions.
Junctional Diversity
Additional diversity is introduced at the junctions of these gene segments. During recombination, we can add or delete nucleotides at the V(D)J junctions, further increasing the variability of the antibody’s antigen-binding site.
Somatic Hypermutation
After B cells encounter an antigen, they can undergo somatic hypermutation, where point mutations are introduced into the variable region genes. This process allows for the selection of B cells producing antibodies with higher affinity for the antigen, a phenomenon known as affinity maturation.
Mechanisms of Antigen Recognition and Binding
Specificity
The unique amino acid sequences and conformations of its CDRs determined the specificity of an antibody for a particular antigen. The shape, charge, and hydrophobicity of the CDRs are complementary to the epitope on the antigen, enabling precise binding.
- Epitope Recognition: An epitope is the specific part of an antigen recognized by an antibody. The fit between the antibody’s CDRs and the antigen’s epitope is often described using the lock-and-key or induced fit models. In the lock-and-key model, the antibody and antigen fit together perfectly without significant conformational changes. In the induced fit model, binding of the antigen induces conformational changes in the antibody, enhancing the binding interaction.
Affinity
Affinity refers to the strength of the binding interaction between an antibody and its antigen. High-affinity antibodies bind their antigens more tightly and are more effective in neutralizing pathogens. Several factors influence antibody affinity:
- CDR3 Length and Composition: The length and amino acid composition of CDR3, particularly in the heavy chain, significantly impact binding affinity. Longer CDR3 regions can create more extensive contact surfaces with the antigen, increasing binding strength.
- Somatic Hypermutation: Following antigen exposure, B cells undergo somatic hypermutation, introducing point mutations in the variable region genes. This process allows for the selection of B cells producing higher-affinity antibodies, a phenomenon known as affinity maturation.
Functional Implications of Antigen Binding
Neutralization of Pathogens
The primary function of antibodies is to neutralize pathogens by binding to their antigens. By specifically recognizing and binding to critical parts of a pathogen, such as viral surface proteins or bacterial toxins, antibodies can prevent these pathogens from infecting host cells or neutralize their toxic effects.
Opsonization and Phagocytosis
Antibody binding can also facilitate the clearance of pathogens through opsonization. Antibodies bound to antigens on the surface of pathogens act as signals for phagocytic cells, such as macrophages and neutrophils, which recognize and ingest the antibody-coated pathogens.
Activation of Complement System
Antibody-antigen complexes can activate the complement system, a series of proteins that enhance the immune response. Complement activation leads to the formation of membrane attack complexes that can lyse pathogens and promote inflammation to recruit additional immune cells to the site of infection.
Applications of Variable Region Engineering in Antibody Design
Humanization and Reduction of Immunogenicity
One of the primary applications of variable region engineering is humanization of antibodies derived from non-human sources, such as mice or rabbits. Non-human antibodies can elicit immune responses when administered in humans, limiting their therapeutic potential. By replacing non-human CDRs with human counterparts through genetic engineering, the resulting antibodies retain their antigen specificity while minimizing immunogenicity. This process involves selecting human-compatible CDRs that maintain or improve binding affinity and therapeutic efficacy, thus enhancing the safety and effectiveness of antibody therapies.
Affinity Maturation for Enhanced Binding Properties
Affinity maturation involves iterative rounds of mutagenesis and selection to optimize antibody binding affinity for a target antigen. This process targets the variable regions, particularly CDRs, where mutations are introduced to enhance antigen-binding interactions. Through techniques such as phage display or yeast display libraries, we can identify and selete variants with improved affinity. Affinity-matured antibodies exhibit higher specificity and potency, making them valuable candidates for therapeutic applications where precise target recognition is critical.
Development of Bispecific and Multispecific Antibodies
Engineering variable regions allows for the creation of bispecific and multispecific antibodies that simultaneously target multiple antigens or cells. By combining variable regions from different antibodies, engineers can design molecules with dual or multiple specificities. This approach is particularly advantageous in cancer immunotherapy, where bispecific antibodies can engage both tumor cells and immune cells, enhancing immune responses against tumors. Multispecific antibodies also offer flexibility in targeting diverse disease pathways or enhancing therapeutic outcomes through synergistic mechanisms.
Antibody-Drug Conjugates (ADCs) for Targeted Therapy
Variable region engineering plays a crucial role in the design of antibody-drug conjugates (ADCs), which combine the specificity of antibodies with the cytotoxic potency of drugs. ADCs deliver cytotoxic agents selectively to cells expressing specific antigens, minimizing systemic toxicity. By optimizing variable regions to ensure precise antigen targeting, ADCs can improve therapeutic efficacy while reducing off-target effects. This targeted approach is particularly effective in treating cancer and other diseases characterized by antigen-specific cell surface markers.
How Do You Find the Variable Region of An Antibody?
Sequence Determination
The first step in identifying the variable region of an antibody is to determine its nucleotide or amino acid sequence. This sequence can be obtained from experimental data (such as sequencing the antibody gene) or retrieved from databases where antibody sequences are stored.
Sequence Alignment and CDR Identification
Within the variable regions, the hypervariable loops known as Complementarity-Determining Regions (CDRs) are identified. CDRs are crucial for antigen recognition and binding specificity. They are typically designated as CDR1, CDR2, and CDR3 for both VH and VL domains.
Structural and Functional Analysis
Further analysis may involve predicting the three-dimensional structure of the antibody variable region using computational modeling techniques. This can provide insights into how the CDRs interact with antigens and other molecules.
Tools and Resources
Various bioinformatics tools and databases are available to aid in these analyses, including:
- IMGT/DomainGapAlign: A tool for aligning antibody sequences and identifying domains (V, D, J, and C).
- IMGT/V-QUEST: A tool for analyzing the immunoglobulin (IG) and T cell receptor (TR) nucleotide sequences.
- Antibody databases: Databases such as IMGT, NCBI, and others store extensive collections of antibody sequences and provide tools for sequence analysis and annotation.
Experimental Validation
Finally, experimental techniques like PCR (Polymerase Chain Reaction) and sequencing validate the identified variable regions and confirm their sequence accuracy. These experimental data complement bioinformatics analyses, providing a comprehensive understanding of the antibody’s variable regions and their role in antigen recognition and binding.
Reference
- Mir, Manzoor. (2020). Immunoglobulins, Magic Bullets and Therapeutic Antibodies.
Read more: Antibody heavy and light chains








